
The effective radiation dose for DXA is considerably lower than the radiation dose for conventional radiography.
This chapter presents general scan positioning and analysis information, but the manufacturers’ specific procedures must be used when actual scans are performed.Ĥ. The numeric bone density results cannot be compared between manufacturers without proper standardization. 39-1), and technologists must be educated about the specific scanner model in their facility. Three major DXA manufacturers are in the United States (see Fig. In conventional radiography, x-ray machines from different manufacturers are operated in essentially the same manner and produce identical images. The referring and interpreting physicians must be skilled in interpreting the clinical and statistical aspects of the numeric density results and relating them to the specific patient.ģ. The images may not be used for diagnosis, and any medical conditions apparent on the image must be followed up by appropriate diagnostic tests. Scan images are only for the purpose of confirming correct positioning of the patient and correct placement of the regions of interest (ROI).
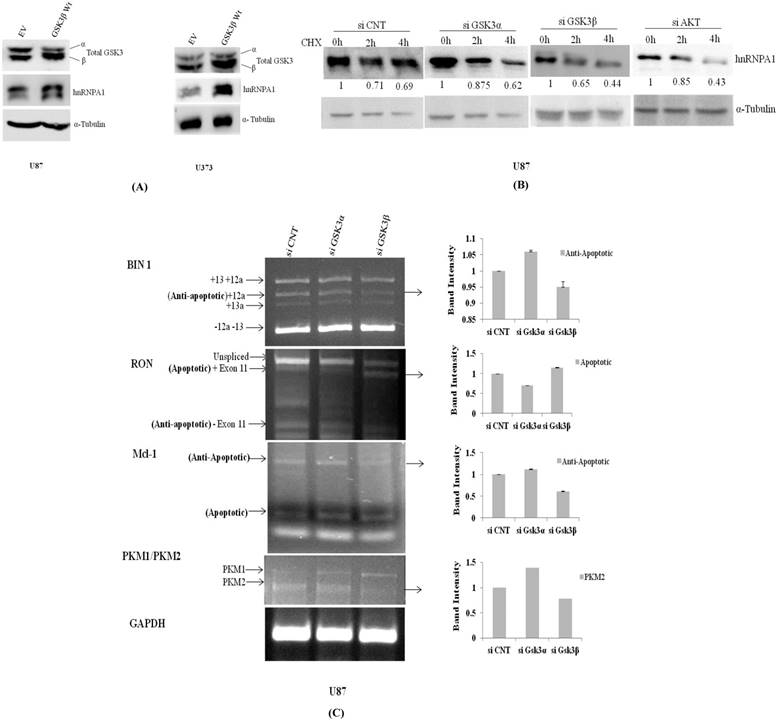
#Densitometry was done with imagej software software
The bone density results are computed by proprietary software from the x-ray attenuation pattern striking the detector, not from the scan image. Having adequate amounts of artifact-free soft tissue is essential to help ensure the reliability of the bone density results.Ģ. The density of the isolated bone is calculated on the basis of the principle that denser, more mineralized bone attenuates (absorbs) more x-ray. This is accomplished by scanning at two different x-ray photon energies (hence the term dual energy x-ray) and mathematically manipulating the recorded signal to take advantage of the differing attenuation properties of soft tissue and bone at the two energies. To quantitate BMD, it is necessary to eliminate the contributions of soft tissue and measure the x-ray attenuation of bone alone.
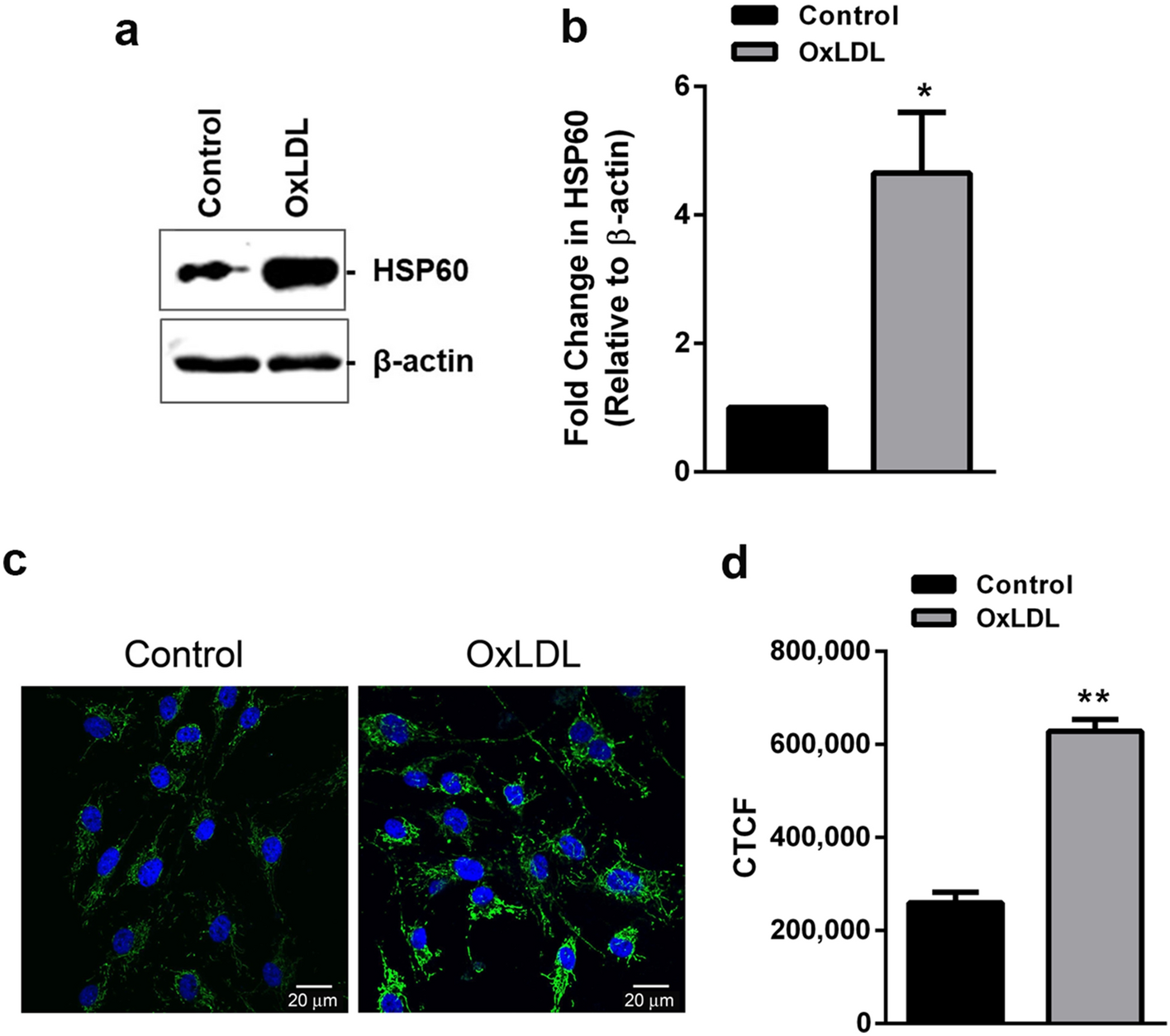
DXA can be conceptualized as a subtraction technique. To our knowledge, this is the first method to reliably quantify nerve fibers through a rapid and automated protocol.1. Because our method can be performed at relatively low cost and in large tissue sections where nerve fibers can be labeled by various antibodies or visualized by expression of reporter proteins, such as green fluorescent protein in transgenic mice, we expect our method to be broadly useful in both research and clinical investigation. The density values are comparable among animals tested, showing a high degree of reproducibility.

Using this method, we have obtained accurate measurements of cholinergic fiber density in hippocampus and a large area of cortex in mouse brain sections immunolabeled with an antibody against the vesicular acetylcholine transporter (VAChT). This allows for time-efficient determination of nerve density and also comparative analysis in large brain structures, such as hippocampus or between various regions of neural circuitry. The combined use of these analytical tools through an automated routine enables reliable detection and quantification of nerve fibers from low magnification, non-uniformly labeled epifluorescence images. Here, we describe an automated and efficient method for nerve fiber quantification, which we developed by making use of widely available software and analytical techniques, including Hessian-based feature extraction in NIH ImageJ and line intensity scan analysis. However, current methods to quantify nerve fibers are resource-intensive and often provide an indirect measurement of nerve fiber density. Quantification of nerve fibers in peripheral and central nervous systems is important for the understanding of neuronal function, organization and pathological changes.


 0 kommentar(er)
0 kommentar(er)
